AZOXYSTROBIN
    (аЧҫъхҘ) (аЧҫъхҘ)
Introduction: Azoxystrobin is a broad spectrum fungicide with activity against several diseases on many edible crops and ornamental plants. Some diseases controlled or prevented are rice blast, rusts, downy mildew, powdery mildew, late blight, apple scab, and Septoria.
Common name: Azoxystrobin
another name: Abound, Amistar, Bankit, Heritage, and Quadris.
Chemical Name: methyl (E)-2-[2-[6-(2-cyanophenoxy)pyrimidin-4-yl]oxyphenyl]-3- methoxyprop-2-enoate
Empirical formula: C22H17N3O5
Structural formula:
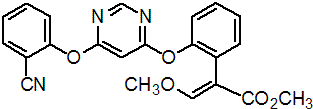
Mol. Weight: 403.394 g/mol
CAS No.: 131860-33-8
Specifications
Leading Azoxystrobin supplier
Azoxystrobin 250 g/L SC
Azoxystrobin 50% WDG
Azoxystrobin 96% TC
Azoxystrobin 98% TC
Packing:
BULK PACKING
Powder: 25KG/Bag, 25KG/Drum, 50KG/Drum etc.
Liquid: 200L/Drum, 20L/Drum, 10L/Drum ect.
SMALL PACKING
Powder: 1kg/Alu bag, 500g/Alu bag, 200g/Alu bag, 100g/Alu bag, 50g/Alu bag, 15g/Alu bag etc.
Liquid: 5L/Drum, 1L/Bottle, 500ml/Bottle, 250ml/Bottle, 100ml/Bottle, 50ml/Bottle etc.
Customerized Packing label
Azoxystrobin FAO standard
Professional registration
HAZARDS IDENTIFICATIONЈә
Hazard statement(s):
H331: Toxic if inhaled
H400: Very toxic to aquatic life
H410: Very toxic to aquatic life with long lasting effects
Precautionary statement(s):
P261: Avoid breathing dust/fume/gas/mist/vapors/spray.
P271: Use only outdoors or in a well-ventilated area.
P273: Avoid release to the environment.
P304+P340: IF INHALED: Remove person to fresh air and keep comfortable for breathing.
P311: Call a POISON CENTER or doctor/...
P321: Specific treatment (see ... on this label).
P391: Collect spillage.
P403+P233: Store in a well-ventilated place. Keep container tightly closed.
P405: Store locked up.
P501: Dispose of contents/container to ...
Supplemental Hazard Statements: none
MAMMALIAN TOXICOLOGY
1) Acute oral LD50 for rat: >5000 a.i.mg/kg. 2) Acute dermal LD50 for rat: >2000 a.i.mg/kg. 3) Inhalation LC50 (4h) for rat: >0.69 a.i. mg/L. 4) Slightly- irritating to skin (rabbits). 5) Slightly- irritating eyes (rabbits). 6) Not a skin sensitiser (guinea pigs).There was no evidence of carcinogenicity, genotoxic, or neurotoxic; azoxystrobin has no effect on fertility parameters nor on foetal or infant development.
ADI (JMPR): 0.2 mg/kg b.w.[2008]
Classification:
Toxicity class WHO (a.i.) U (Unlikely to present an acute hazard)
EC Risk Classification:T - Toxic: R23; N - Dangerous for the environment: R50, R53
ECOTOXICOLOGY
Effects on birds: Abamectin is practically nontoxic to birds. Acute oral LD50 for Bobwhite quail is >2000 a.i.mg/kg. There were no adverse effects on reproduction when mallard ducks were fed dietary doses of 3, 6, or 12 ppm for 18 weeks. Effects on aquatic organisms: Abamectin is highly toxic to fish and extremely toxic to aquatic invertebrates. The LC50 (96 h) is 0.0036 mg/L in rainbow trout, 0.0096 mg/L in bluegill sunfish, 0.015 mg/L in sheepshead minnows, 0.024 mg/L in channel catfish, and 0.042 mg/L in carp. The LC50 (48h) in Daphnia magna, a small freshwater crustacean, is 0.003 mg/L. The LC50 (96h) for is 0.0016 mg/L in pink shrimp, 430 mg/L in eastern oysters, and 153 mg/L in blue crab. Abamectin did not bioaccumulate in bluegill sunfish exposed to 0.099 ug/L for 28 days in a flow-through tank. The levels in fish were from 52 to 69 times the ambient water concentration, indicating that abamectin does not accumulate or persist in fish. Effects on bees: organisms: Abamectin is highly toxic to bees, with contact LC50(48h) is 0.002 ҰМg/bee and an oral LD50 of 0.009 ҰМg/bee. Effects on Algae: Acute EC50(72h), growth is > 1.59mg/L. Effects on earthworms: moderate toxicity to earthworms, Acute 14 day LC50 is 33 mg kg.
ENVIRONMENTAL FATE
Azoxystrobin's production may result in its release to the environment through various waste streams; its use as a fungicide will result in its direct release to the environment. If released to air, a vapor pressure of 8.25ЎБ10-13 mm Hg at 25Ўж indicates azoxystrobin will exist solely in the particulate phase in the atmosphere. Particulate-phase azoxystrobin will be removed from the atmosphere by wet or dry deposition. If released to soil, azoxystrobin is expected to have moderate to low mobility based upon Koc values of 207 to 594. Volatilization from moist soil surfaces is not expected to be an important fate process based upon an estimated Henry's Law constant of 7.3ЎБ10-14 atm-cu m/mole. Azoxystrobin was present at 22.3 and 60.9% of applied dose (100 mg/kg) to compost aged for 3 and 12 months, respectively, in 125-day tests, suggesting that biodegradation is not an important environmental fate process in soil. If released into water, azoxystrobin is expected to adsorb to suspended solids and sediment based upon the Koc values. In the dark and under aerobic conditions, the half-life of azoxystrobin was in the range of eight to 12 weeks. Volatilization from water surfaces is not expected to be an important fate process based upon this compound's estimated Henry's Law constant. An estimated BCF of 21 suggests the potential for bioconcentration in aquatic organisms is low. Azoxystrobin has been experimentally shown to be stable to hydrolysis at pH 5 and 7, at pH 9 a half-life of 12.1 days is reported. Occupational exposure to azoxystrobin may occur through inhalation of dust and dermal contact with this compound at workplaces where azoxystrobin is produced or used. Monitoring data indicate that the general population may be exposed to azoxystrobin via ingestion of food containing residual and dermal contact in places azoxystrobin is used.
Usage:
The molecule was synthetised for the first time by Dr. Christopher Godfrey at Jealott's Hill International Research Centre in Bracknell. Controls the following pathogens: Erysiphe graminis, Puccinia spp., Leptosphaeria nodorum, Septoria tritici and Pyrenophora teres on temperate cereals; Pyricularia oryzae and Rhizoctonia solani on rice; Plasmopara viticola and Uncinula necator on vines; Sphaerotheca fuliginea and Pseudoperonospora cubensis on cucurbitaceae; Phytophthora infestans and Alternaria solani on potato and tomato; Mycosphaerella arachidis, Rhizoctonia solani and Sclerotium rolfsii on peanut; Monilinia spp. and Cladosporium carpophilum on peach; Pythium spp. and Rhizoctonia solani on turf; Mycosphaerella spp. on banana; Cladosporium caryigenum on pecan; Colletotrichum spp. and Guignardia citricarpa on citrus; Colletotrichum spp. and Hemileia vastatrix on coffee. Phytotoxicity Good crop safety, except on some varieties of apple (e.g. McIntosh, Cox).
Application:
Biochemistry Inhibits mitochondrial respiration by blocking electron transfer between cytochrome b and cytochrome c1, at the ubiquinol oxidising site. Controls pathogenic strains resistant to the 14-demethylase inhibitors, phenylamides, dicarboxamides or benzimidazoles. Mode of action Fungicide with protectant, curative, eradicant, translaminar and systemic properties. Inhibits spore germination and mycelial growth, and also shows antisporulant activity. Uses Controls the following pathogens at application rates between 100 to 375 g/ha: Erysiphe graminis, Puccinia spp., Leptosphaeria nodorum, Septoria tritici and Pyrenophora teres on temperate cereals; Pyricularia oryzae and Rhizoctonia solani on rice; Plasmopara viticola and Uncinula necator on vines; Sphaerotheca fuliginea and Pseudoperonospora cubensis on cucurbitaceae; Phytophthora infestans and Alternaria solani on potato and tomato; Mycosphaerella arachidis, Rhizoctonia solani and Sclerotium rolfsii on peanut; Monilinia spp. and Cladosporium carpophilum on peach; Pythium spp. and Rhizoctonia solani on turf; Mycosphaerella spp. on banana; Cladosporium caryigenum on pecan.
| 






