Isoprothiolane
    өҫОББй өҫОББй
Introduction: Isoprothiolane, also known as diisopropyl 1,3-dithiolan-2- ylidenemalonate or ipt, belongs to dicarboxylic acids and derivatives class of compounds. Those are organic compounds containing exactly two carboxylic acid groups. Isoprothiolane is practically insoluble (in water) and an extremely weak basic (essentially neutral) compound (based on its pKa). Isoprothiolane can be synthesized from 1,3-dithiolane and malonic acid. Isoprothiolane can also be synthesized into isoprothiolane sulfoxide. Within the cell, isoprothiolane is primarily located in the membrane (predicted from logP). Isoprothiolane is an Agricultural fungicide for rice crop.
Common name: Isoprothiolane
Another name: Fudiolan; Fujione; Fuji 1; IPT (pesticide); etc.
Chemical name: diisopropyl 1,3-dithiolan-2-ylidenemalonate
Empirical formula: C12H18O4S2
Structural formula:
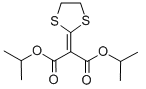
Mol. Weight: 290.40 g/mol
CAS No.: 50512-35-1
Specifications
Leading Isoprothiolane supplier
Isoprothiolane 95% TC
Isoprothiolane 98% TC
Isoprothiolane 40% EC
Isoprothiolane 40% WP
Packing:
BULK PACKING
Powder: 25kg/Bag, 25kg/Drum, 50kg/Drum etc.
Liquid: 200L/Drum, 20L/Drum, 10L/Drum etc.
SMALL PACKING
Powder: 1kg/Alu bag, 500g/Alu bag, 200g/Alu bag, 100g/Alu bag, 50g/Alu bag, 15g/Alu bag etc.
Liquid: 5L/Drum, 1L/Bottle, 500ml/Bottle, 250ml/Bottle, 100ml/Bottle, 50ml/Bottle etc.
Customerized packing label
Isoprothiolane FAO standard
Professional registration
HAZARDS IDENTIFICATION
Hazard statement(s)
H302 (100%): Harmful if swallowed.
H302 (100%): Harmful if swallowed.
Precautionary statement(s)
P264: Wash ... thoroughly after handling.
P270: Do not eat, drink or smoke when using this product.
P301+P312: IF SWALLOWED: call a POISON CENTER/doctor/... IF you feel unwell.
P330: Rinse mouth.
P501: Dispose of contents/container to an approved waste disposal plant.
Supplemental Hazard Statements: none.
MAMMALIAN TOXICOLOGY
Acute toxicity: 1) Acute oral LD50 for rats is >300 mg/kg. 2) Acute dermal LD50 for rats is >2000 mg/kg. 3) Acute inhalation toxicity LC50 (4 h) for rats is >2.32 mg/L. 4) Skin irritation: Non-irritating to skin (rabbits). 5) Eye irritation: Slightly irritating to eyes (rabbits). 6) Skin sensitization for guinea pig: Dermal sensitizer (maximization test).
NOEL: (2 y) for rats is 10.9 mg/kg/day; (18 m) for mice is 95.6 mg/kg/day; (52 w) for dogs is 10 mg/kg/day. Other Not carcinogenic. Not genotoxic.
ADI (JMPR) 0-0.1 mg/kg b.w. [2017]
Classification:
WHO Classification: II (Moderately hazardous)
EC Risk Classification: Not classified: Obsolete
US EPA Classification (formulation): III (Caution - Slightly toxic)
ECOTOXICOLOGY
Effect on birds: Acute oral LD50 for Japanese quail is >4180 mg/kg. Effect on fish: Acute LC50 (96 h) for Rainbow trout is 6.8 mg/l. Effects on aquatic invertebrates: Acute EC50 (48 h) for Daphnia magna is 62 mg/l. Effects on algae: Acute 72 hour EC50 for Pseudokirchneriella subcapitata is 4.58 mg/l. Effects on earthworms: Acute 14 day LC50 is >91.95 mg/kg.
ENVIRONMENTAL FATE
Isoprothiolane was shown to be hydrolytically stable at pH 4 and 7 over 5 days at 50ЎА0.5Ўж. At pH 9, isoprothiolane degrades above 10% after 5 days at 50Ўж. Isoprothiolane monoester was the major hydrolytic degradate in basic solutions. DT 50 values in solution at pH 9 of isoprothiolane at 40, 50 and 60Ўж were 147, 26 and 9.0 days, respectively. Thus, the estimated DT50 values at 20Ўж and 25Ўж were > 1000 and > 3000 days, respectively. Isoprothiolane is considered to be hydrolytically stable at environmental temperatures at all pH values. The degradation rate of isoprothiolane in soils under artificial light irradiation and in dark controls was determined based on the percent isoprothiolane present in the extracts. DT50 and DT90 values for the net photodegradation of isoprothiolane were 28 and 92 days, respectively. The degradation rate of the metabolite monosulfoxide was as 13 and 42 days for the DT50 and DT90, respectively.
Usage: Isoprothiolane was introduced by Nihon Nohyaku Co., Ltd. It is an insecticide and fungicide used to control a range of rice pests.
Application: Biochemistry Inhibits penetration and elongation of infection hyphae, by inhibiting formation of infection peg or cellulase secretion. Mode of action Systemic fungicide with protective and curative action, absorbed by the leaves and roots, with translocation acropetally and basipetally. The mobility in rice plants of various analogues reported (M. Uchida,Pestic. Biochem. Physiol.,1980, 14, 249). Uses Controls the following pathogens and insect pests, at 400-600 g/ha (foliar spray), 3.0-6.0 g/nursery box (spreading treatment), 3.6-6.0 kg/ha (submerged treatment), 360-600 g/tree (soil incorporation): Pyricularia oryzae, Helminthosporium sigmoideum, Fusarium nivale on rice; Rosellinia necatrix on pome fruit, stone fruit and grapes; Delphacidae (planthoppers) on rice. Also used on rice to accelerate rooting, promote root elongation and control non-parasitic damping-off.
| 






