BROMOXYNIL OCTANOATE
    РБхЈдеұҪлж РБхЈдеұҪлж
Introduction: An HBN herbicide used for post-emergence control of annual broad-leaved weeds including annual sowthistle, mustard, cocklebur, pennycress, ragweed, groundsel, henbit, russian thistle, velvetleaf, morning glory, lambsquarter, kochia, wild buckweed on cereals including wheat, barley, oats, rye; flax; sweetcorn; field corn; onions & garlic; turf.
Common name: Bromoxynil Octanoate
Another name: Bromoxynil octanoate [ISO], Bromoxynil octanoic acid ester, etc.
Chemical name: 2,6-dibromo-4-cyanophenyl octanoate
Empirical formula: C15H17Br2NO2
Structural formula:
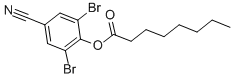
Mol. Weight: 403.10 g/mol
CAS No.: 1689-99-2
Specifications
Leading Bromoxynil Octanoate supplier
Bromoxynil Octanoate 250 g/L EC
Bromoxynil Octanoate 300 g/L EC
Bromoxynil Octanoate 95% TC
Packing:
BULK PACKING
Solid: 25kg/Bag, 25kg/Drum, 50kg/Drum etc.
Liquid: 200L/Drum, 20L/Drum, 10L/Drum ect.
SMALL PACKING
Solid: 1kg/Alu bag, 500g/Alu bag, 200g/Alu bag, 100g/Alu bag, 50g/Alu bag, 15g/Alu bag etc.
Liquid: 5L/Drum, 1L/Bottle, 500ml/Bottle, 250ml/Bottle, 100ml/Bottle, 50ml/Bottle etc.
Customerized packing label
Bromoxynil Octanoate FAO standard
Professional registration
HAZARDS IDENTIFICATION
Hazard statement(s)
H302: Harmful if swallowed.
H317: May cause an allergic skin reaction.
H331: Toxic if inhaled.
H361d ***: Suspected of damaging the unborn child.
H400: Very toxic to aquatic life.
H410: Very toxic to aquatic life with long lasting effects.
Precautionary statement(s)
P201: Obtain special instructions before use.
P202: Do not handle until all safety precautions have been read and understood.
P261: Avoid breathing dust/fume/gas/mist/vapors/spray.
P264: Wash ... thoroughly after handling.
P270: Do not eat, drink or smoke when using this product.
P271: Use only outdoors or in a well-ventilated area.
P272: Contaminated work clothing should not be allowed out of the workplace.
P273: Avoid release to the environment.
P280: Wear protective gloves/protective clothing/eye protection/face protection.
P281: Use personal protective equipment as required.
P301+P312: IF SWALLOWED: call a POISON CENTER/doctor/... IF you feel unwell.
P302+P352: IF ON SKIN: wash with plenty of water.
P304+P340: IF INHALED: Remove person to fresh air and keep comfortable for breathing.
P308+P313: IF exposed or concerned: Get medical advice/attention.
P311: Call a POISON CENTER or doctor/...
P321: Specific treatment (see ... on this label).
P330: Rinse mouth.
P333+P313: IF SKIN irritation or rash occurs: Get medical advice/attention.
P363: Wash contaminated clothing before reuse.
P391: Collect spillage.
P403+P233: Store in a well-ventilated place. Keep container tightly closed.
P405: Store locked up.
P501: Dispose of contents/container to ...
Supplemental Hazard Statements: none
MAMMALIAN TOXICOLOGY
Acute toxicity: 1) Acute oral LD50 for rat: >141 a.i.mg/kg. 2) Acute dermal LD50 for rat: >2000 a.i.mg/kg. 3) Inhalation LC50 (4 h) for rat: 0.72 a.i.mg/L. 4) Non-irritating to skin (rabbits). 5) Non-irritating to eyes (rabbits). 6) A skin sensitiser (guinea pigs).
NOEL: (90 d) for rats is 15.6, dogs is 5 mg/kg daily.
ADI: 0.01 mg/kg b.w./day [Mouse, 18 months, SF=100]
Classification:
Toxicity class WHO (a.i.): II (Moderately hazardous)
US EPA Classification (formulation): III (Caution - Slightly toxic)
EC Risk Classification: Reproduction risk category 3: R63; T - Toxic: R23; Xn - Harmful: R22, R43; N - Dangerous for the environment: R50, R53
ECOTOXICOLOGY
Effect on birds: moderate toxicity to birds, acute oral LD50 for Bobwhite quail is 170 a.i.mg/kg. Effect on fish: high toxicity to fish, acute 96 hour LC50 for Rainbow trout is 0.041 a.i.mg/L. Effect on aquatic invertebrates: high toxicity to aquatic invertebrates, acute 48 hour EC50 for Daphnia magna is 0.044 a.i.mg/L. Effect on algae: low toxicity to algae, acute 72 hour EC50 for Pseudokirchneriella subcapitata is >28.0 a.i.mg/L. Effect on honeybees: low toxicity to honeybees, contact acute 48 hour LD50 is >100 a.i.ҰМg/bee; oral acute 48 hour LD50 is >119.8 a.i.ҰМg/bee. Effect on earthworms: moderate toxicity to earthworms, acute 14 day LC50 for Eisenia foetida is 45 a.i.mg/kg.
ENVIRONMENTAL FATE
Bromoxynil octanoate's production may result in its release to the environment through various waste streams; its use as a herbicide will result in its direct release to the environment. If released to air, a vapor pressure of 4.8ЎБ10-6 mm Hg at 25 deg C indicates bromoxynil octanoate will exist in both the vapor and particulate phases in the ambient atmosphere. Vapor-phase bromoxynil will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals; the half-life for this reaction in air is estimated to be 2.2 days. Particulate-phase bromoxynil octanoate will be removed from the atmosphere by wet and dry deposition. If released to soil, bromoxynil octanoate is expected to have no mobility based upon an estimated Koc of 21,000. In an aged soil leaching study, bromoxynil octanoate residues were found to not be mobile in four soils and aquatic sediment. A Kd of 7 mL/g (Koc = 1,003) was reported in soils with 1.2% organic matter. Volatilization from moist soil surfaces is expected based upon an estimated Henry's Law constant of 3.2ЎБ10-5 atm-cu m/mole. However, adsorption to soil is expected to attenuate volatilization. Bromoxynil octanoate is not expected to volatilize from dry soil surfaces based upon its vapor pressure. Hydrolysis, photolytic degradation, and microbially-mediated degradation are important environmental processes for bromoxynil octanoate. 14C Labeled bromoxynil octanoate was reported to degrade in sandy loam soil with a half-life of 2 days; the major degradation product was CO2, which accounted for 64.28% of the applied radioactivity after 90 days. In soil photolysis experiments, bromoxylnil octanoate was found to degrade with a half-life of 2.6 days when irradiated with a xenon arc lamp; a degradation half-life of 3.6 days was observed in a dark control. Degradation in the dark control suggests that processes in addition to photolysis (e.g., hydrolysis or microbial degradation) were also occurring. Field dissipation half-lives ranging from 0.5 to 28 days have been reported. If released into water, bromoxynil octanoate is expected to adsorb to suspended solids and sediment based upon the estimated and reported Koc values. Bromoxynil octanoate added to sandy loam soil flooded with pond water degraded with a half-life of <12 hours under aerobic conditions at 25 deg C in the dark. Bromoxynil octanoate was reported to degrade with a half-life of 3.7 days in a sandy loam sediment under anaerobic conditions. Volatilization from water surfaces is expected to occur based upon this compound's estimated Henry's Law constant. Estimated volatilization half-lives for a model river and model lake are 2.5 and 25 days, respectively. However, volatilization from water surfaces is expected to be attenuated by adsorption to suspended solids and sediment in the water column. The volatilization half-life from a model pond is about 130 years when adsorption is considered. In addition, the rapid hydrolysis of bromoxynil octanoate may attenuate its volatilization from water surfaces. Hydrolysis half-lives of 34.1, 11.5, and 1.7 days at pH 5, 7, and 9 have been reported for bromoxynil octanoate. A BCF of 230 (whole fish) was reported in bluegill sunfish when continuously exposed to 14C radiolabeled bromoxynil octanoate at 1.3-4.6 ug/L. This BCF suggests the potential for bioconcentration in aquatic organisms is high, provided the compound is not metabolized by the organism. Occupational exposure to bromoxynil octanoate may occur through inhalation and dermal contact with this compound at workplaces where bromoxynil octanoate is produced or used. (SRC)
Usage: Herbicidal properties of bromoxynil described independently by R. L. Wain (Nature (London), 1963, 200, 28), by K. Carpenter & B. J. Heywood (ibid., p. 28), and by Amchem Products Inc. Development reviewed by B. J. Heywood (Chem. Ind. (London), 1966, p. 1946). Introduced by May & Baker Ltd and by Amchem Products Inc. (both now Bayer CropScience). Patents: GB 1067033 to May & Baker; US 3397054; US 4332613 both to Amchem. Manufacturers: Bayer CropScience Nufarm Ltd; Hesenta; Makhteshim-Agan; Punjab; Sundat; Zhejiang. Photosynthetic electron transport inhibitor at the photosystem II receptor site; also uncouples oxidative phosphorylation.
Application: Selective contact herbicide with some systemic activity. Absorbed by the foliage, with limited translocation. Post-emergence control of annual broad-leaved weeds, especially young seedlings of the Polygonaceae, Compositae, and certain Boraginaceae, in cereals, ryegrass-seed crops, turf, and non-crop land, at rates up to 450 g octanoate/ha; in maize and sorghum, applied at up to 600 g/ha. Often used in combination with other herbicides, to extend the spectrum of control.
| 






