 |
| Ш§Щ„ШұШҰЩҠЩҖШіЩҠШ© - وسيطة |
| Name Of Product |
CAS NO. |
Specification |
MSDS |
| Isobutyronitrile |
78-82-0 |
98%TC |

|
|
|
Isobutyronitrile Basic information
|
|
Product Name:
|
Isobutyronitrile
|
|
Synonyms:
|
Isobutylronitrile;#nisoЈn-Butyronitrile;ISOBUTYRONITRILE, 99.6%;Azobis (2-methylpropionitrile);1-Cyano-1-methylethane;2-Cyanopropane;2-methyl-propanenitril;2-methyl-propanoicacinitrile
|
|
CAS:
|
78-82-0
|
|
MF:
|
C4H7N
|
|
MW:
|
69.11
|
|
EINECS:
|
201-147-5
|
|
Product Categories:
|
Organics
|
|
Mol File:
|
78-82-0.mol
|
|
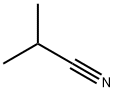
|
|
|
|
Isobutyronitrile Chemical Properties
|
|
Melting point
|
-72 ЎгC (lit.)
|
|
Boiling point
|
107-108 ЎгC (lit.)
|
|
density
|
0.770 g/mL at 20 ЎгC (lit.)
|
|
vapor density
|
2.38 (vs air)
|
|
vapor pressure
|
100 mm Hg ( 54.4 ЎгC)
|
|
refractive index
|
n20/D 1.372(lit.)
|
|
Fp
|
39 ЎгF
|
|
storage temp.
|
Flammables area
|
|
solubility
|
slightly soluble in water and acetone, very soluble in alcohol and ether
|
|
form
|
Liquid
|
|
color
|
Clear colorless to light yellow
|
|
Water Solubility
|
35 g/L (20 ºC)
|
|
Merck
|
14,5156
|
|
BRN
|
1340512
|
|
Stability:
|
Stable. Flammable. May form explosive mixtures with air. Incompatible with strong oxidizing agents.
|
|
CAS DataBase Reference
|
78-82-0(CAS DataBase Reference)
|
|
NIST Chemistry Reference
|
Propanenitrile, 2-methyl-(78-82-0)
|
|
EPA Substance Registry System
|
Isobutyronitrile (78-82-0)
|
|
|
|
Isobutyronitrile Usage And Synthesis
|
|
Chemical Properties
|
colourless liquid
|
|
Uses
|
Solvent.
|
|
Uses
|
Isobutyronitrile can be derived from isobutyraldehyde and is used in organic synthesis and as a gasoline additive.
|
|
Production Methods
|
Isobutyronitrile is prepared from isobutyraldehyde by cyanation with ammonia.
|
|
General Description
|
A clear colorless liquid. Flash point 47ЎгF. Less dense than water. Vapors heavier than air. Toxic oxides of nitrogen produced during combustion.
|
|
Air & Water Reactions
|
Highly flammable. Slightly soluble in water.
|
|
Reactivity Profile
|
Isobutyronitrile is incompatible with the following: Oxidizers, reducing agents, strong acids & bases .
|
|
Hazard
|
Toxic by ingestion, inhalation, and skin absorption.
|
|
Health Hazard
|
Isobutyronitrile is considered highly hazardous and full precautions should be taken to prevent skin contact or inhalation of vapor. Inhaled isobutyronitrile is about 2.4 times as toxic as acetonitrile in rats. In order to protect workers, the recommended TWA limit is obtained by dividing that for acetonitrile by the factor 2.4. NIOSH has therefore recommended that employee exposure should not exceed 8 p.p.m. (22 mg/m3) for either compound as a TLV-TWA .
Isobutyronitrile was implicated in several cases of industrial poisoning. Thiess and Hey reported that a worker became unconscious following isobutyronitrile exposure. His immediate symptomatology included convulsive movements of upper limbs, soft and thready pulse, dilated pupils, shallow and gasping breathing and secretion of viscous mucus. After hospital admission, an exacerbation of the condition occurred with tonic-clonic movements of the upper extremities. The patient was cyanotic, the pulse faint and thready. The patient was treated intravenously with noradrenaline (1 mg) followed by amyl nitrite, sodium nitrite, and sodium thiosulphate. The man's cyanotic condition diminished and his pulse strengthened, although his gasping breath and upper limb convulsions continued. He was given intravenous injections of lobeline and phenobarbital. Rapid improvement occurred and the patient recovered gradually, leaving the hospital symptom-free at 14 d after admission. Two milder inhalation exposures of isobutyronitrile were reported by Zeller et al in which an unknown concentration of vapor produced headache, dizziness, and vomiting at 10-60 min after exposure. The intensity of symptoms varied with the concentration and duration of exposure.
|
|
Health Hazard
|
Poisonous; may be fatal if inhaled, swallowed, or absorbed through skin. Contact may cause burns to skin and eyes. (Non-Specific -- Nitriles) Primarily, they are skin and eye irritants. Large doses cause collapse and stop breathing.
|
|
Fire Hazard
|
Vapor may explode if ignited in an enclosed area. Toxic oxides of nitrogen are produced during combustion. Isobutyronitrile is a flammable/combustible material and may be ignited by heat, sparks, or flames. Vapors may travel to a source of ignition and flash back. Container may explode in heat of fire. Vapor explosion and poison hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Hazardous polymerization may not occur.
|
|
Industrial uses
|
Isobutyronitrile is used in organic synthesis, as a catalyst in the polymerization of ethylene and in the petroleum industry as a gasoline additive.
|
|
Metabolism
|
Thiocyanate was present in the urine of rats dosed orally with isobutyronitrile.
|
|
Purification Methods
|
Shake the nitrile with conc HCl (to remove isonitriles), then with water and aqueous NaHCO3. After a preliminary drying with silica gel or Linde type 4A molecular sieves, it is shaken or stirred with CaH2 until hydrogen evolution ceases, then decanted and distilled from P2O5 (not more than 5g/L, to minimize gel formation) or Drierite (b 101-103o/760mm). Finally it is refluxed with, and slowly distilled from CaH2 (5g/L), taking precautions to exclude moisture. [Beilstein 2 H 294, 2 I 129, 2 II 263, 2 III 655, 2 IV 853.]
|
|
|
|
Isobutyronitrile Preparation Products And Raw materials
|

|
|
|
|







