Tetramethrin 胺菊酯
Introduction: Tetramethrin is a potent multi-purpose insecticide often used to control insects presenting risks to public health such as mosquitoes, flies, cockroaches, wasps, hornets, fleas, ants in houses and public health situations.
Common name: Tetramethrin
Another name: Phthalthrin, Neopinamine, Neo-pynamin, Neopinamin, Bioneopynamin, Insectol, Neopynamin forte, Py-Kill, Tetramethrin [INN], Tetramethrine, Tetralate, d-Phthalthrin, Weo-Pynamin, Sumitomo SP-1103, Tetramethrinum, Tetrametrina, Tetramethrin racemic.
Chemical name: cyclohex-1-ene-1,2-dicarboximidomethyl (1RS,3RS;1RS,3SR)-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate
Empirical formula: C19H25NO4
Structural formula:
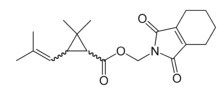
Mol. Weight: 331.406 g/mol
CAS No.: 7696-12-0
Specifications
Leading Tetramethrin supplier
Tetramethrin 20 g/L EC
Tetramethrin 95% TC
Packing:
BULK PACKING
Powder: 25kg/Bag, 25kg/Drum, 50kg/Drum etc.
Liquid: 200L/Drum, 20L/Drum, 10L/Drum ect.
SMALL PACKING
Powder: 1kg/Alu bag, 500g/Alu bag, 200g/Alu bag, 100g/Alu bag, 50g/Alu bag, 15g/Alu bag etc.
Liquid: 5L/Drum, 1L/Bottle, 500mL/Bottle, 250mL/Bottle, 100mL/Bottle, 50mL/Bottle etc.
Customerized Packing label
Professional registration
HAZARDS IDENTIFICATION
Hazard statement(s)
H320: Causes eye irritation.
H371: May cause damage to organs.
H400: Very toxic to aquatic life.
H410: Very toxic to aquatic life with long lasting effects.
Precautionary statement(s)
P260: Do not breathe dust/fume/gas/mist/vapors/spray.
P264: Wash ... thoroughly after handling.
P270: Do not eat, drink or smoke when using this product.
P273: Avoid release to the environment.
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do - continue rinsing.
P309+P311: IF exposed or if you feel unwell: call a POISON CENTER or doctor/physician.
P337+P313: IF eye irritation persists: Get medical advice/attention.
P391: Collect spillage.
P405: Store locked up.
P501: Dispose of contents/container to ...
Supplemental Hazard Statements: none
MAMMALIAN TOXICOLOGY
Acute toxicity: 1) Acute oral LD50 for rat: >5000 a.i.mg/kg. 2) Acute dermal LD50 for rabbit: >2000 a.i.mg/kg. 3) Inhalation LC50 (4 h) for rat: >2.73 a.i. mg/L. 4) Non- irritating to skin (rabbits). 5) Non-irritating to eyes (rabbits). 6)Not a skin sensitiser (guinea pigs). NOEL In 13 w feeding trials, dogs receiving 5000 mg/kg diet showed no ill-effects. In 6 mo feeding trials, no-effect level for rats was 1500 mg/kg diet. Other No evidence of oncogenicity.
ADI: 0.02 mg/kg b.w./day
Classification:
Toxicity class WHO (a.i.): U (Unlikely to present an acute hazard)
US EPA Classification (formulation): IV (Caution - Not acutely toxic)
EC Risk Classification: N - Dangerous for the environment: R50/52
ECOTOXICOLOGY
Effect on birds: low toxicity to birds, acute oral LD50 for Bobwhite quail is >2250 a.i.mg/kg. Effect on fish: high toxicity to fish, acute 96 hour LC50 for Rainbow trout is 0.016 a.i.mg/L. Effect on aquatic invertebrates: high toxicity to aquatic invertebrates, acute 48 hour EC50 for Daphnia magna is 0.045 a.i.mg/L. Effect on honeybees: high toxicity to honeybees, unknown mode acute 48 hour LD50 is >0.16a.i.μg/bee.
ENVIRONMENTAL FATE
Tetramethrin's production may result in its release to the environment through various waste streams; its use as an insecticide will result in its direct release to the environment. If released to air, a vapor pressure of 7.08×10-6 mm Hg at 30 deg C indicates tetramethrin will exist in both the vapor and particulate phases in the atmosphere. Vapor-phase tetramethrin will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals; the half-life for this reaction in air is estimated to be 3 hours. The half-life for the reaction in air with ozone is estimated to be 30 minutes. Particulate-phase tetramethrin will be removed from the atmosphere by wet or dry deposition. Tetramethrin contains chromophores that absorb at wavelengths >290 nm and therefore may be susceptible to direct photolysis by sunlight. If released to soil, tetramethrin is expected to have low mobility based upon an estimated Koc of 790. Volatilization from moist soil surfaces is expected to be an important fate process based upon an estimated Henry's Law constant of 1.7×10-6 atm-cu m/mole. Tetramethrin is not expected to volatilize from dry soil surfaces based upon its vapor pressure. Although environmental biodegradation data specific to tetramethrin were not available, the pyrethroid class of insecticides is degraded readily by environmental microorganisms. If released into water, tetramethrin is expected to adsorb to suspended solids and sediment based upon the estimated Koc. Volatilization from water surfaces is expected to be an important fate process based upon this compound's estimated Henry's Law constant. Estimated volatilization half-lives for a model river and model lake are 950 hours and 290 days, respectively. An estimated BCF of 34 suggests the potential for bioconcentration in aquatic organisms is moderate, provided the compound is not metabolized by the organism. Tetramethrin is susceptible to alkaline hydrolysis.
Usage: History Introduced by Sumitomo Chemical Co., Ltd and later by FMC Corp. (who no longer manufacture or market it). First registered in Japan in 1964. Patents JP 453929; JP 462108; US 3268398 all to Sumitomo Manufacturers Agro-Chemie; Changzhou Kangmei; Endura; Jiangsu Yangnong; Sumitomo.
Application: Biochemistry Acts on the nervous system of insects, disturbs the function of neurons by interaction with the sodium channel. Mode of action Non-systemic insecticide with contact action. Gives rapid knockdown. Uses Normally used in combination with synergists (e.g. piperonyl butoxide) and other insecticides for control of flies, cockroaches, mosquitoes, wasps, and other insect pests in public health and home and garden use.
| 






