Isoproturon 异丙隆
Introduction: Isoproturon is is a selective herbicide belonging to the family of substituted ureas and acts principally after root absorption, either pre-emergence or post emergence. It is effective against several broad-leaved weeds and numerous grassy weeds. It is safe to all varieties of soft winter wheat, barley and rye.
Common name: Isoproturon
Another name: Graminon; 3-(4-Isopropylphenyl)-1,1-dimethylurea; Tolkan; Arelon; Belgran; Arelon R; Nocilon; IPU Stefes; IP-Flo; Alon; Hytane 500L; DPX 6774; Zodiac TX; etc.
Chemical name: 3-(4-isopropylphenyl)-1,1-dimethylurea
Empirical formula: C12H18N2O
Structural formula:
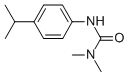
Mol. Weight: 206.28 g/mol
CAS No.: 34123-59-6
Specifications
Leading Isoproturon supplier
Isoproturon 95% TC
Isoproturon 50% WP
Isoproturon 482 g/L SC
Packing:
BULK PACKING
Powder: 25kg/Bag, 25kg/Drum, 50kg/Drum etc.
Liquid: 200L/Drum, 20L/Drum, 10L/Drum etc.
SMALL PACKING
Powder: 1kg/Alu bag, 500g/Alu bag, 200g/Alu bag, 100g/Alu bag, 50g/Alu bag, 15g/Alu bag etc.
Liquid: 5L/Drum, 1L/Bottle, 500ml/Bottle, 250ml/Bottle, 100ml/Bottle, 50ml/Bottle etc.
Customerized packing label
Isoproturon FAO standard
Professional registration
HAZARDS IDENTIFICATION
Hazard statement(s)
H332 (11.92%): Harmful if inhaled.
H351 (100%): Suspected of causing cancer.
H400 (100%): Very toxic to aquatic life.
H410 (100%): Very toxic to aquatic life with long lasting effects.
Precautionary statement(s)
P201: Obtain special instructions before use.
P202: Do not handle until all safety precautions have been read and understood.
P261: Avoid breathing dust/fume/gas/mist/vapors/spray.
P271: Use only outdoors or in a well-ventilated area.
P273: Avoid release to the environment.
P281: Use personal protective equipment as required.
P304+P312: IF INHALED: Call a POISON CENTER/doctor/... if you feel unwell.
P304+P340: IF INHALED: Remove person to fresh air and keep comfortable for breathing.
P308+P313: IF exposed or concerned: Get medical advice/attention.
P312: Call a POISON CENTER or doctor/... if you feel unwell.
P391: Collect spillage.
P405: Store locked up.
P501: Dispose of contents/container to an approved waste disposal plant.
Supplemental Hazard Statements: none.
MAMMALIAN TOXICOLOGY
Acute toxicity: 1) Acute oral LD50 for rats is 1826 mg/kg. 2) Acute dermal LD50 for rats is >2000 mg/kg. 3) Acute inhalation toxicity LC50 (4 h) for rats is 1.95 mg/L. 4) Skin irritation: Non-irritating to skin (rabbits). 5) Eye irritation: Non-irritating to eyes (rabbits). 6) Skin sensitization for guinea pig: Non-sensitizing.
NOEL: (2 y) for rats is 3.1 mg/kg/day. Other Not genotoxic. No indications of delayed neurotoxicity in the hen or standard tests.
ADI 0-0.015 mg/kg b.w.
Classification:
WHO Classification: II (Moderately hazardous)
EC Risk Classification: Carcinogen category 3: R40; N - Dangerous for the environment: R50, R53
US EPA Classification (formulation): No consensus across products or no products available
ECOTOXICOLOGY
Effect on birds: Acute oral LD50 for Bobwhite quail is 1401 mg/kg. Effect on fish: Acute LC50 (96 h) for Rainbow trout is 37 mg/l. Effects on aquatic invertebrates: Chronic 21 day NOEC for Daphnia magna is 0.12 mg/l. Effects on algae: Acute 72 hour EC50 for Navicula pelliculosa is 0.013 mg/l. Effects on bees: contact acute 48 hour LD50 is 200 μg/bee, oral acute 48 hour LD50 is 195 μg/bee. Effects on earthworms: Acute 14 day LC50 is >1000 mg/kg.
ENVIRONMENTAL FATE
Animals In rats, following oral administration, 50% is eliminated within the first 8 h, predominantly in the urine. Plants In plants, degradation is mainly via hydroxylation of the isopropyl group to 1,1-dimethyl-3-[4-(2'-hydroxy-2'-propyl)phenyl]urea; N-dealkylation also occurs. Soil/Environment Undergoes enzymic and microbial demethylation at the nitrogen, and hydrolysis of the phenylurea to 4-isopropylaniline. DT50 in soil 6-28 d; rate of degradation increases 3x between 10℃ and 30℃(sandy soil) and 10x in an organic soil over the same temperature range.
Usage: Isoproturon was introduced by Ciba-Geigy AG (became Novartis Crop Protection AG). It is a herbicide for use, typically, in cereals to control annual grasses and many broad-leaved weeds.
Application: Biochemistry Photosynthetic electron transport inhibitor at the photosystem II receptor site. Mode of action Selective systemic herbicide, absorbed by the roots and leaves, with translocation. Uses Pre- and post-emergence control of annual grasses (Alopecurus myosuroides, Apera spica-venti, Avena fatua and Poa annua) and many annual broad-leaved weeds in spring and winter wheat (except durum wheat), spring and winter barley, winter rye, and triticale, at 1.0-1.5 kg/ha.
| 






