ATRAZINE
      莠去津, 阿特拉津 莠去津, 阿特拉津
Introduction: A triazine herbicide used pre- and post-emergence with restricted permitted uses to control broad-leaved weeds and grasses including morning glory, barnyard grass, cocklebur, lambsquarters, crabgrass, pigweed, buckwheat, ragweed, foxtail in corn, sorghum, sugarcane, turf and asparagus.
Common name: Atrazine
Another name: Gesaprim, Chromozin, Oleogesaprim, Aktikon, Argezin, Atazinax, Atranex, Atrasine, Atrazin, Fenamin, Fenatrol, Gesoprim, Hungazin, Pitezin, Primatol, Primaze, Radazin, Strazine, Zeazine, Aatrex, Candex, Cyazin, Inakor, Vectal, Wonuk, Zeazin,Crisatrina, Crisazine, Fenamine, Aatram, Akticon, Atrataf, Atratol, Griffex, Radizin, Zeapos, Atred, Atrex , etc.
Chemical name: 6-chloro-N2-ethyl-N4-isopropyl-1,3,5-triazine-2,4-diamine
Empirical formula: C8H14ClN5
Structural formula:
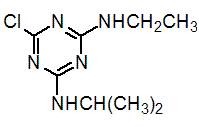
Mol. Weight: 215.68 g/mol
CAS No.: 1912-24-9
Specifications
Leading Atrazine supplier
Atrazine 500 g/L SC
Atrazine 80% WP
Atrazine 90% WDG
Atrazine 95% TC
Atrazine 97% TC
Packing:
BULK PACKING
Solid: 25kg/Bag, 25kg/Drum, 50kg/Drum etc.
Liquid: 200L/Drum, 20L/Drum, 10L/Drum ect.
SMALL PACKING
Solid: 1kg/Alu bag, 500g/Alu bag, 200g/Alu bag, 100g/Alu bag, 50g/Alu bag, 15g/Alu bag etc.
Liquid: 5L/Drum, 1L/Bottle, 500ml/Bottle, 250ml/Bottle, 100ml/Bottle, 50ml/Bottle etc.
Customerized packing label
Atrazine FAO standard
Professional registration
HAZARDS IDENTIFICATION
Hazard statement(s)
H317: May cause an allergic skin reaction.
H373: Causes damage to organs through prolonged or repeated exposure.
H410: Very toxic to aquatic life with long lasting effects.
Precautionary statement(s)
P260: Do not breathe dust/fume/gas/mist/vapors/spray.
P261: Avoid breathing dust/fume/gas/mist/vapors/spray.
P272: Contaminated work clothing should not be allowed out of the workplace.
P273: Avoid release to the environment.
P280: Wear protective gloves/protective clothing/eye protection/face protection.
P302+P352: IF ON SKIN: wash with plenty of water.
P314: Get medical advice/attention if you feel unwell.
P321: Specific treatment (see ... on this label).
P333+P313: IF SKIN irritation or rash occurs: Get medical advice/attention.
P363: Wash contaminated clothing before reuse.
P391: Collect spillage.
P501: Dispose of contents/container to ...
Supplemental Hazard Statements: none
MAMMALIAN TOXICOLOGY
Acute toxicity: 1) Acute oral LD50 for rat: 1869 a.i.mg/kg. 2) Acute dermal LD50 for rat: >3100 a.i.mg/kg. 3) Inhalation LC50 (4 h) for rat: 5.8 a.i.mg/L. 4) Mild skin irritant (rabbits). 5) Non-irritating to eyes (rabbits). 6) skin sensitiser (guinea pigs).
NOEL: (2 y) for rats is 10 mg/kg diet (0.5 mg/kg daily), for dogs is 150 mg/kg diet (3.75 mg/kg daily), for mice is 10 mg/kg diet (1.4 mg/kg daily).
ADI (JMPR): 0.02 mg/kg b.w. [2007]
Classification:
Toxicity class WHO (a.i.): III (Slightly hazardous)
US EPA Classification (formulation): III (Caution - Slightly toxic)
EC Risk Classification: Xn - Harmful: R48/22, R43; N - Dangerous for the environment: R50, R53
ECOTOXICOLOGY
Effect on birds: low toxicity to birds, acute oral LD50 for Japanese quail is 4237 a.i.mg/kg. Effect on fish: moderate toxicity to fish, acute 96 hour LC50 for Rainbow trout is 4.5 a.i.mg/L. Effect on aquatic invertebrates: moderate toxicity to aquatic invertebrates, acute 48 hour EC50 for Daphnia magna is 85 a.i.mg/L. Effect on algae: moderate toxicity to algae, acute 72 hour EC50 for Raphidocelis subcapitata is 0.059 a.i.mg/L. Effect on honeybees: low toxicity to honeybees, contact acute 48 hour LD50 is >100 a.i.μg/bee, oral acute 48 hour LD50 is >100 a.i.μg/bee. Effect on earthworms: moderate toxicity to earthworms, acute 14 day LC50 is 79 a.i.mg/kg.
ENVIRONMENTAL FATE
If released to air, a vapor pressure of 2.89×10-7 mm Hg at 25 deg C indicates atrazine will exist in both the vapor and particulate phases in the atmosphere. Vapor-phase atrazine will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals; the half-life for this reaction in air is estimated to be 14 hours. Particulate-phase atrazine will be removed from the atmosphere by wet or dry deposition. If released to soil, atrazine is expected to have high to slight mobility based upon a Koc range of 54 to 1164. Volatilization half-lives of atrazine in soil range from 111 to >1000 days. Biodegradation of atrazine in soil is affected by the moisture content of the soil. The half-life of atrazine at 25 deg C in wet (and dry) Colorado loam soil, New York sandy loam soil, and Mississippi silt loam was determined to be 30 (90), 28 (55), and 35 (78) days, respectively. Temperature and pH also affect the biodegradation of atrazine in soil. Microbial degradation of atrazine in silty loam and sand samples occurred in alkaline soil with the primary metabolites of desethyl atrazine and deisopropylatrazine while chemical degradation of atrazine yielded hydroxyatrazine; in addition, atrazine degraded 3-4 times faster in soils at 25 deg C than at 10 deg C. In soils, hydrolysis of atrazine is favored by low soil pH, high organic matter content, low moisture content, high temperature, and high clay content. If released into water, atrazine may adsorb to suspended solids and sediment in water based on the Koc value range. Atrazine was degraded by 23% in raw seawater after 96 hours. However, atrazine was found to be recalcitrant in natural groundwater after a period of 96 days. The half-life of atrazine in an anaerobic wetland sediment was determined to be 224 days with no additional carbon present; the only metabolite present during degradation was hydroxyatrazine. Volatilization from water surfaces is not expected to be an important fate process based on a Henry's Law constant of 2.6×10-9 atm-cu. A BCF range of <0.27 to 100 in fish suggests bioconcentration in aquatic organisms is low to moderate. Hydrolysis of atrazine follows first-order kinetics, producing hydroxyatrazine as the major transformation product. Atrazine may hydrolyze fairly rapidly in either acidic or basic environments, yet is fairly resistant to hydrolysis at neutral pHs. The rate of hydrolysis was found to increase drastically upon small additions of humic materials, indicating atrazine hydrolysis could be catalyzed in natural waters. Atrazine may undergo photolysis in sunlit surface waters.
Usage: Herbicide reported by H. Gysin & E. Knüsli (Proc. Int. Congr. Crop Prot., 4th, Hamburg, 1957). Introduced by J. R. Geigy S.A. (now Syngenta AG). Patents: BE 540590; GB 814947. Manufacturers: Agrochem; Atanor; Crystal; Dow AgroSciences; Drexel; DuPont; Hegang Heyou; Hesenta; Makhteshim-Agan; Meghmani; Nagarjuna Agrichem; Oxon; Rallis; Sannong; Sharda; Syngenta. Photosynthetic electron transport inhibitor at the photosystem II receptor site. Maize tolerance is attributed to rapid detoxification by glutathione transferases.
Application: Selective systemic herbicide, absorbed principally through the roots, but also through the foliage, with translocation acropetally in the xylem and accumulation in the apical meristems and leaves. Pre- and post-emergence control of annual broad-leaved weeds and annual grasses in maize, sorghum, sugar cane, pineapples, chemical fallow, grassland, macadamia nuts, conifers, industrial weed control. In Europe, use is concentrated in maize and sorghum at £1.5 kg/ha. Used also in combinations with many other herbicides. Phytotoxicity Phytotoxic to many crops, including most vegetables, potatoes, soya beans, and peanuts.
|







