GIBBERELLIC ACID(GA3)
  赤霉素(GA3) 赤霉素(GA3)
Introduction: A plant growth regulator with a variety of uses including improving fruit set, increasing berry size and controlling fruit maturity.
Common name: Gibberellic Acid(GA3)
Another name: Gibberellin, Gibreskol, Brellin, Cekugib, Grocel, Gibefol, Regulex, Gibberellins, Berelex, Gibberellinsaeure, etc.
Chemical name: (3S,3aS,4S,4aS,7S,9aR,9bR,12S)-7,12-dihydroxy-3-methyl-6-methylene-2-oxoperhydro-4a,7-methano-9b,3-propenoazuleno[1,2-b]furan-4-carboxylic acid
Empirical formula: C19H22O6
Structural formula:
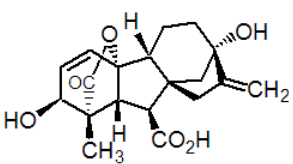
Mol. Weight: 346.37 g/mol
CAS No.: 77-06-5
Specifications
Leading Gibberellic Acid(GA3) supplier
Gibberellic Acid(GA3) 40 g/L SL
Gibberellic Acid(GA3) 10% TB
Gibberellic Acid(GA3) 20% TB
Gibberellic Acid(GA3) 90% TC
Gibberellic Acid(GA3) 95% TC
Packing:
BULK PACKING
Solid: 25kg/Bag, 25kg/Drum, 50kg/Drum etc.
Liquid: 200L/Drum, 20L/Drum, 10L/Drum ect.
SMALL PACKING
Solid: 1kg/Alu bag, 500g/Alu bag, 200g/Alu bag, 100g/Alu bag, 50g/Alu bag, 15g/Alu bag etc.
Liquid: 5L/Drum, 1L/Bottle, 500ml/Bottle, 250ml/Bottle, 100ml/Bottle, 50ml/Bottle etc.
Customerized packing label
Gibberellic Acid(GA3) FAO standard
Professional registration
HAZARDS IDENTIFICATION
Hazard statement(s)
H319 (100%): Causes serious eye irritation.
Precautionary statement(s)
P264: Wash ... thoroughly after handling.
P280: Wear protective gloves/protective clothing/eye protection/face protection.
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do - continue rinsing.
P313: Get medical advice/attention.
Supplemental Hazard Statements: none
MAMMALIAN TOXICOLOGY
Acute toxicity: 1) Acute oral LD50 for rat: >5000 a.i.mg/kg. 2) Acute dermal LD50 for rat: >2000 a.i.mg/kg. 3) Inhalation LC50 (4 h) for rat: >4.49 a.i.mg/L. 4) Non-irritating to skin (rabbits). 5) Non-irritating to eyes (rabbits). 6) Skin sensitiser: not applicable.
NOEL: (90 d) for rats and dogs is >1000 mg/kg diet (6 d/w).
ADI: 0.55 mg/kg b.w./day [Rat, SF=100]
Classification:
Toxicity class WHO (a.i.): U (Unlikely to present an acute hazard)
US EPA Classification (formulation): III (Caution - Slightly toxic)
ECOTOXICOLOGY
Effect on birds: low toxicity to birds, acute oral LD50 for Bobwhite quail is >2250 a.i.mg/kg. Effect on fish: low toxicity to fish, acute 96 hour LC50 for Rainbow trout is >120 a.i.mg/L. Effect on aquatic invertebrates: moderate toxicity to aquatic invertebrates, acute 48 hour EC50 for Daphnia magna is 76 a.i.mg/L. Effect on algae, low toxicity to algae, acute 72 hour EC50 for Pseudokirchneriella subcapitata is 17.0 a.i.mg/L. Effect on honeybees: moderate toxicity to honeybees, contact acute 48 hour LD50 is >25 a.i.μg/bee. Effect on earthworms: low toxicity to earthworms, acute 14 day LC50 for Eisenia foetida is >1250 a.i.mg/kg.
ENVIRONMENTAL FATE
Gibberellic acid's production and use as a plant growth regulator will result in its direct release to the environment. Gibberellic acid is produced by several molds, principally Gibberella fujikuroi. If released to air, an estimated vapor pressure of 2.1×10-13 mm Hg at 25 deg C indicates gibberellic acid will exist solely in the particulate phase in the atmosphere. Particulate-phase gibberellic acid will be removed from the atmosphere by wet or dry deposition. If released to soil, gibberellic acid is expected to have very high mobility based upon an estimated Koc of 32. The pKa of gibberellic acid is 4, indicating that this compound will primarily exist in anion form in the environment and anions generally do not adsorb to organic carbon and clay more strongly than the undissociated acid. Volatilization from moist soil surfaces is not expected to be an important fate process based upon an estimated Henry's Law constant of 1.6×10-15 atm-cu m/mole. Volatilization of gibberellic acid is attenuated by the ionic form. Gibberellic acid is not expected to volatilize from dry soil surfaces based upon its vapor pressure. Degradation of gibberellic acid in soil is approximately 85% in five days and >90% in ten days incubated at 24 deg C in the dark. If released into water, gibberellic acid is not expected to adsorb to suspended solids and sediment based upon the estimated Koc. Volatilization from water surfaces is not expected to be an important fate process based upon this compound's estimated Henry's Law constant. A pKa of 4 indicates gibberellic acid will exist almost entirely in an anion form at pH values of 5 to 9. An estimated BCF of 3 suggests the potential for bioconcentration in aquatic organisms is low.
Usage: Discovered by E. Kurosawa (Trans. Nat. Hist. Soc. (Formosa), 1926, 16, 213), who called it gibberellin A. Later ICI Plant Protection Ltd (now Syngenta AG) isolated a compound with similar biological properties and chemical structure, and this was called gibberellic acid. It and other members of the gibberellin group (over 70 are known) occur naturally in a wide variety of plant species. The establishment of its chemical structure and stereochemistry has been reviewed (J. F. Grove, Q. Rev. Chem. Soc., 1961, 15, 56; B. E. Cross et al., Adv. Chem. Series, 1961, No. 28, p. 13). Introduced by ICI (now Syngenta AG). Manufacturers: Abbott; JIE; Jingma; Nufarm SA; Sharda; Syngenta.
Application: Acts as a plant growth regulator on account of its physiological and morphological effects in extremely low concentrations. Generally affects only the plant parts above the soil surface. Has a variety of applications, e.g. to improve fruit setting of clementines and pears (especially William pears); to loosen and elongate clusters and increase berry size in grapes; to control fruit maturity by delaying development of the yellow colour in lemons; to reduce rind stain and retard rind ageing in navel oranges; to counteract the effects of cherry yellows virus diseases in sour cherries; to produce uniform seedling growth in rice; to promote elongation of winter celery crop; to induce uniform bolting and increase seed production in lettuce for seed; to break dormancy and stimulate sprouting in seed potatoes; to extend the picking season by hastening maturity in artichokes; to increase the yield in forced rhubarb; to increase the malting quality of barley; to produce brighter-coloured, firmer fruit, and to increase the size of sweet cherries; to increase yields and aid harvesting of hops; to reduce internal browning and increase yields of Italian prunes; to increase fruit set and yields of tangelos and tangerines; to improve fruit setting in blueberries; to advance flowering and increase the yield of strawberries; and also a variety of applications on ornamentals. Application rates up to 80 g/a per application, depending on desired effect.
| 






