Chlorfenapyr
    溴虫腈 溴虫腈
Introduction: Chlorfenapyr can control of many species of insects and mites, including those resistant to carbamate, organophosphate and pyrethroid Insecticides and also chitin-synthesis inhibitors, in cotton, vegetables, citrus, top fruit, vines and soya beans. Among pests resistant to conventional products which are controlled by Chlorfenapyr are Brevipalpus phoenicis (leprosis mite), Leptinotarsa decemlineata (Colorado potato beetle), Helicoverpa spp., Heliothis spp., Plutella xylostella (diamond-back moth) and Tetranychus spp. Chlorfenapyr’s use in resistance management programmes for control of various cotton pests is under evaluation.
Common name: Chlorfenapyr
Another name: Pirate, Kotetsu, Pylon, Pirate 3F
Chemical name: 4-bromo-2-(4-chlorophenyl)-1-ethoxymethyl-5-trifluoromethyl-1H-pyrrole-3-carbonitrile
Empirical formula: C15H11BrClF3N2O
Structural formula:
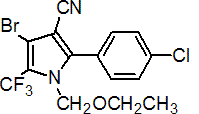
Mol. Weight: 407.62 g/mol
CAS No.: 122453-73-0
Specifications
Leading Chlorfenapyr supplier
Chlorfenapyr 100 g/L EC
Chlorfenapyr 240 g/L EC
Chlorfenapyr 98% TC
Packing:
BULK PACKING
Solid: 25kg/Bag, 25kg/Drum, 50kg/Drum etc.
Liquid: 200L/Drum, 20L/Drum, 10L/Drum ect.
SMALL PACKING
Solid: 1kg/Alu bag, 500g/Alu bag, 200g/Alu bag, 100g/Alu bag, 50g/Alu bag, 15g/Alu bag etc.
Liquid: 5L/Drum, 1L/Bottle, 500ml/Bottle, 250ml/Bottle, 100ml/Bottle, 50ml/Bottle etc.
Customerized packing label
Chlorfenapyr FAO standard
Professional registration
HAZARDS IDENTIFICATION
Hazard statement(s)
H302: Harmful if swallowed.
H331: Toxic if inhaled.
H400: Very toxic to aquatic life.
H410: Very toxic to aquatic life with long lasting effects.
Precautionary statement(s)
P261, P264, P270, P271, P273, P301+P312, P304+P340, P311, P321, P330, P391, P403+P233, P405, and P501
P261: Avoid breathing dust/fume/gas/mist/vapors/spray.
P264: Wash ... thoroughly after handling.
P270: Do not eat, drink or smoke when using this product.
P271: Use only outdoors or in a well-ventilated area.
P273: Avoid release to the environment.
P301+P312: IF SWALLOWED: call a POISON CENTER/doctor/... IF you feel unwell.
P304+P340: IF INHALED: Remove person to fresh air and keep comfortable for breathing.
P311: Call a POISON CENTER or doctor/...
P330: Rinse mouth.
P391: Collect spillage.
P403+P233: Store in a well-ventilated place. Keep container tightly closed.
P405: Store locked up.
P501: Dispose of contents/container to ...
Supplemental Hazard Statements: none
MAMMALIAN TOXICOLOGY
Acute toxicity: 1) Acute oral LD50 for rat: 441 a.i.mg/kg. 2) Acute dermal LD50 for rabbit: >2000 a.i.mg/kg. 3) Inhalation LC50 (4 h) for rat: 1.9 a.i. mg/L. 4) Non- irritating to skin (rabbits). 5) Moderate-irritating to eyes (rabbits). 6) Not a skin sensitiser (guinea pigs). No mutagenic effect was found in various tests with microorganisms and mammalian cell culture. The substance was not mutagenic in a test with mammals. In long-term studies in rats and mice in which the substance was given by feed, a carcinogenic effect was not observed. No indications of a developmental toxic / teratogenic effect were seen in animal studies.
ADI (JMPR): 0.03 mg/kg b.w.[2013]
Classification:
Toxicity class WHO (a.i.): II (Moderately hazardous)
US EPA Classification (formulation): III (Caution - Slightly toxic)
EC Risk Classification: T - Toxic: R23; Xn - Harmful: R22; N - Dangerous for the environment: R50, R53
ECOTOXICOLOGY
Effect on birds: high toxicity to birds, acute oral LD50 for Bobwhites quail is 10 a.i.mg/kg. Effect on fish: high toxicity to fish, acute 96 hour LC50 for Rainbow trout is 0.007 a.i.mg/L. Effect on aquatic invertebrates: high toxicity to aquatic invertebrates, acute 48 hour EC50 for Daphnia magna is 0.0061 a.i.mg/L. Effect on honeybees: high toxicity to honeybees, acute 48 hour LD50 is 0.12 a.i.μg/bee.
ENVIRONMENTAL FATE
Chlorfenapyr's production may result in its release to the environment through various waste streams; it's use as an insecticide and an acaricide will result in its direct release to the environment. If released to air, an estimated vapor pressure of 7.4×10-8 mm Hg at 25 deg C indicates chlorfenapyrwill exist in both the vapor and particulate phases in the atmosphere. Vapor-phase chlorfenapyr will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals; the half-life for this reaction in air is estimated to be 1.2 days. Particulate-phase chlorfenapyrwill be removed from the atmosphere by wet or dry deposition. If released to soil, chlorfenapyr is expected to have no mobility based upon an estimated Koc of 10,000. Volatilization from moist soil surfaces is not expected to be an important fate process based upon an estimated Henry's Law constant of 5.7×10-9 atm-cu m/mole. Biodegradation half-life of chlorfenapyr in soil is 230-250 days and in sediment is 250 days. Based on these half-lives biodegradation of chlorfenapyr is not expected to be an important environmental fate process. If released into water, chlorfenapyris expected to adsorb to suspended solids and sediment based upon the estimated Koc. Chlorfenapyr photodegrades in sterile fresh water with a half-life of 5-7 days therefore photolysis is expected to be the principal fate of chlorfenapyr in water. Volatilization from water surfaces is not expected to be an important fate process based upon this compound's estimated Henry's Law constant. BCFs of 83-114 in bluegill sunfish suggest the potential for bioconcentration in aquatic organisms is moderate to high. Hydrolysis of chlorfenapyr is not expected to be environmentally relevant given a half-life of >30 days. Occupational exposure to chlorfenapyr may occur through dermal contact with this compound at workplaces where chlorfenapyr is produced or used. The general population may be exposed to chlorfenapyr via ingestion of food, and dermal contact with this compound near fields where chlorfenapyr is applied. (SRC)
Usage: History Developed by American Cyanamid Co. (now BASF AG). Manufacturers: BASF.
Application: Biochemistry Oxidative removal in vivo of the N-ethoxymethyl group generates the active species, which is a mitochondrial uncoupler. Insecticide and acaricide with mainly stomach and some contact action. Exhibits good translaminar but limited systemic activity in plants. Uses Control of many species of insects and mites, including those resistant to carbamate, organophosphate and pyrethroid insecticides and also chitin-synthesis inhibitors, in cotton, vegetables, citrus, top fruit, vines and soya beans. Among pests resistant to conventional products which are controlled by chlorfenapyr are Brevipalpus phoenicis (leprosis mite), Leptinotarsa decemlineata (Colorado potato beetle), Helicoverpa spp., Heliothis spp., Plutella xylostella (diamond-back moth) and Tetranychus spp. Also control of many species of structural and household Formicidae (especially Camponotus, Iridomyrmex, Monomorium, and Solenopsis), Blattellidae (especially Blatta, Blattella, Periplaneta and Supella spp.), Kalotermitidae (especially Incisitermes) and Rhinotermitidae (especially Reticulitermes, Coptotermes, Heterotermes) at use rates of between 0.125 to 0.50% a.i. w/w. No phytotoxicity observed at field use rates.
| 






