DINOTEFURAN
   
Introduction: Dinotefuran is an insecticide of the neonicotinoid class developed by Mitsui Chemicals for control of insect pests such as aphids, whiteflies, thrips, leafhoppers, leafminers, sawflies, mole cricket, white grubs, lacebugs, billbugs, beetles, mealybugs, and cockroaches on leafy vegetables, in residential and commercial buildings, and for professional turf management. Its mechanism of action involves disruption of the insect's nervous system by inhibiting nicotinic acetylcholine receptors. In order to avoid harming beneficial insects such as bees, it should not be applied during bloom.
Common name: Dinotefuran
Another name: 1-Methyl-2-nitro-3-((tetrahydrofuran-3-yl)methyl)guanidine, Albarin, Mikeblock, MTI 446, N''-methyl-N-nitro-N'-((tetrahydro-3-furanyl)methyl)guanidine
Chemical Name: 2-methyl-1-nitro-3-[(tetrahydro-3-furanyl) methyl] guanidine
Empirical formula: C7H14N4O3
Structural formula:
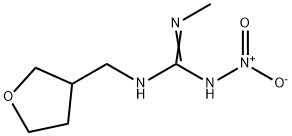
Mol. Weight: 202.21 g/mol
CAS No.: 165252-70-0
Specifications
Leading Dinotefuran supplier
Dinotefuran 20% SG
Dinotefuran 20% WP
Dinotefuran 75% WDG
Dinotefuran 95% TC
Dinotefuran 98% TC
Packing:
BULK PACKING
25KG/Bag, 25KG/Drum, 50KG/Drum etc.
SMALL PACKING
1kg/Alu bag, 500g/Alu bag, 200g/Alu bag, 100g/Alu bag, 50g/Alu bag, 15g/Alu bag etc.
Customerized Packing label
Dinotefuran FAO standard
Professional registration
HAZARDS IDENTIFICATION
Hazard statement(s)
H302: Harmful if swallowed.
Precautionary statement(s)
P264: Wash ... thoroughly after handling.
P270: Do not eat, drink or smoke when using this product.
P301+P312: IF SWALLOWED: call a POISON CENTER/doctor/... IF you feel unwell.
P330: Rinse mouth.
P501: Dispose of contents/container to ...
Supplemental Hazard Statements: none
MAMMALIAN TOXICOLOGY
Acute toxicity: 1) Acute oral LD50 for rat: >2000 a.i.mg/kg. 2) Acute dermal LD50 for rat: >2000 a.i.mg/kg. 3) Inhalation LC50 (4 h) for rat: >4.09 a.i. mg/L. 4) Slightly-irritating to skin (rabbits). 5) Moderate-irritating to eyes (rabbits). 6) Not a skin sensitiser (guinea pigs). No adverse effects in fetuses were seen in the developmental toxicity studies in rats or rabbits, at maternally toxic doses, and offspring effects in the reproduction study. There was a qualitative increase in sensitivity in rat pups in the reproductive toxicity study. Not likely to be carcinogenic to humans.There is no concern for mutagenicity resulting from exposure to dinotefuran.
ADI (JMPR): 0.22 mg/kg b.w.
Classification:
EC Risk Classification: Xn - Harmful: R22; Xi - Irritant: R36; N - Dangerous for the environment: R53, R56, R57.
ECOTOXICOLOGY
Effect on birds: low toxicity to birds, acute oral LD50 for Coturnix japonica is >2000 a.i.mg/kg. Effect on fish: low toxicity to fish, acute 96 hour LC50 for rainbow trout is >100 a.i.mg/L. Effect on aquatic invertebrates: low toxicity to aquatic invertebrates, acute 48 hour EC50 for Daphnia magna is >968.3 a.i.mg/L. Effect on algae: low toxicity to algae, acute 72 hour EC50 for Pseudokirchneriella subcapitata is >100 a.i.mg/L. Effect on honeybees: high toxicity to honeybees, contact acute 48 hour LD50 is >0.023 a.i.μg/bee. Effect on earthworms: high toxicity to earthworms, acute 14 day LC50 for Eisenia foetida is 4.9 a.i.mg/kg.
ENVIRONMENTAL FATE
Dinotefuran's production may result in its release to the environment through various waste streams; it's use as an insecticide for leafy vegetables (except Brassica), and for use in professional turf management, professional ornamental production, and in the residential indoor, pet, lawn and garden markets will result in its direct release to the environment. If released to air, a vapor pressure of 1.3×10-8 mm Hg at 25 deg C indicates dinotefuran will exist solely in the particulate phase in the atmosphere. Particulate-phase dinotefuran will be removed from the atmosphere by wet or dry deposition. Dinotefuran undergoes photolysis in aqueous solution indicating that it may be susceptible to direct photolysis in the atmosphere. If released to soil, dinotefuran is expected to have very high mobility based upon Koc values in the range of 6 to 45 measured in various soils. Volatilization from moist soil surfaces is not expected to be an important fate process based upon an estimated Henry's Law constant of 6.4×10-14 atm-cu m/mole. Dinotefuran is not expected to volatilize from dry soil surfaces given its vapor pressure. The mean aerobic biodegradation half-life of dinotefuran measured in 2 soils was 81.5 days. If released into , dinotefuran is not expected to adsorb to suspended solids and sediment based upon the range of Koc values. Volatilization from water surfaces is not expected to be an important fate process based upon this compound's estimated Henry's Law constant. Dinotefuran was stable to hydrolysis over the pH range of 4 to 9, but was rapidly photolyzed in aqueous solution with a half-life of 1.8 days. An estimated BCF of 3 suggests the potential for bioconcentration in aquatic organisms is low. Occupational exposure to dinotefuran may occur through inhalation of dust and dermal contact with this compound at workplaces where dinotefuran is produced or used.
Usage: Dinotefuran was developed by Mitsui Group to control insect pests in rice, leafy vegetables and fruit.
Application: Dinotefuran is a broad-spectrum insecticide, which is used to controls insect pests such as aphids, whiteflies, thrips, leafhopper, leafminer, sawfly, mole cricket, white grubs, lacebugs, billbugs, beetles, mealybugs, sawfly larvae, and cockroaches in leafy vegetables, in residential and commercial buildings, outdoor uses for professional turf management, turf farms, professional ornamental production, golf courses, residential indoor, lawn and garden use. Mode of action Active by ingestion and by contact; also exhibits root-systemic activity. Uses Controls a range of hemipterous and other pests, at 100-200 g/ha.
| 






